MRI scanning for patients with CIEDs requires some additional steps to be taken both prior to imaging, and on attendance. Growing experience with over 10,000 MRI scans in device patients has allowed a number of guidelines to be established.
Guidelines can be found below (access may be required):
2017 French Society of Cardiovascular Imaging Protocol
2017 International Heart Rhythm Society
2015 German Roentgen Society Statement
2014 Canadian Position Statement
2013 European Society of Cardiology
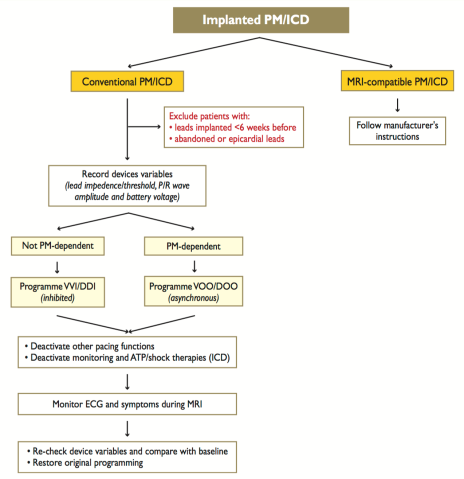
Non-MRI conditional device guidelines:
‘Assessing the Risks Associated with MRI in Patients with a Pacemaker or Defibrillator’ (The MagnaSafe registry) N Engl J Med 2017; 376:755-764
MagnaSafe registry protocol
‘Safety of Magnetic Resonance Imaging in patients with cardiac devices’
2017 N Engl J Med 377;26
